Written on: February 28, 2013 by W. Stephen Tait
Hello everyone. One of the most difficult questions to answer about corrosion is why it’s so random. For example, you have a static storage test on a new formula or line extension, and you open twelve sample containers after six months and you find:
The formula in the containers was from the same batch, so the chemical composition is the same; all the containers came from the same pallet (i.e., manufactured at the same time); and the containers were all filled at the same time. Frustrating!
It would be even more frustrating if you found eleven corroded containers and one pristine, corrosion-free container. Why isn’t the corrosion more consistent?
The answer is complex because a) the surface metallurgy of spray package metals is very complex; b) laminate film, polymer and tin coatings do not bond uniformly with the complex metal surface; c) there are many different types of spray package corrosion; and d) the initiation of corrosion is often a complex chain of events resulting from interactions between your formula and the container.
Let’s explore the metallurgical reasons for spray package corrosion inconsistency.
Corrosion Basics: Metal surfaces & coated metal surfaces are not homogeneous
The Figure on the facing page contains a scanning electron micrograph that shows the metallurgical structure details of the steel used to fabricate tinplated steel aerosol containers. The aluminum used to fabricate aerosol containers and laminated film bags have a similar structure.
The metal in the Figure was prepared by removing the tin coating, mechanical polishing and then etching the steel with an acidic solution to show the metallurgical structure. The larger shapes are referred to as grains. There are also inclusions, precipitates, grain boundaries and crystal defects identified in the Figure.
Notice in the Figure that there are several different grain sizes and shapes. Notice also that there are different grain heights, plus different thicknesses and heights for grain boundaries. The variety of heights and thicknesses resulted from different grains and grain boundaries having different corrosion rates in the etching solution.
Thus the Figure demonstrates that non-uniform corrosion results in part from the complex mixture of the different types of materials and metallurgy on the metal surface. Certain formula compositions will also corrode the different steel and aluminum grains at different rates (e.g., a disinfectant formula verses an aqueous air freshener formula).
Theoretically, polymer coatings and laminate films will bond with all the different materials on the package metal surface identified in the Figure. However, polymer coatings and laminate films actually bond differently with the different types of grains, inclusions, precipitates, grain boundaries and crystal defects.
Some of the polymer-surface-material bonds will be very strong and others will be very weak. For example, polymer and laminate films bonds with precipitates are typically weaker than the corresponding bonds with metal grains. Consequently, the polymer coating and laminate film will also have a random variety of different bonds on the spray package metal surface.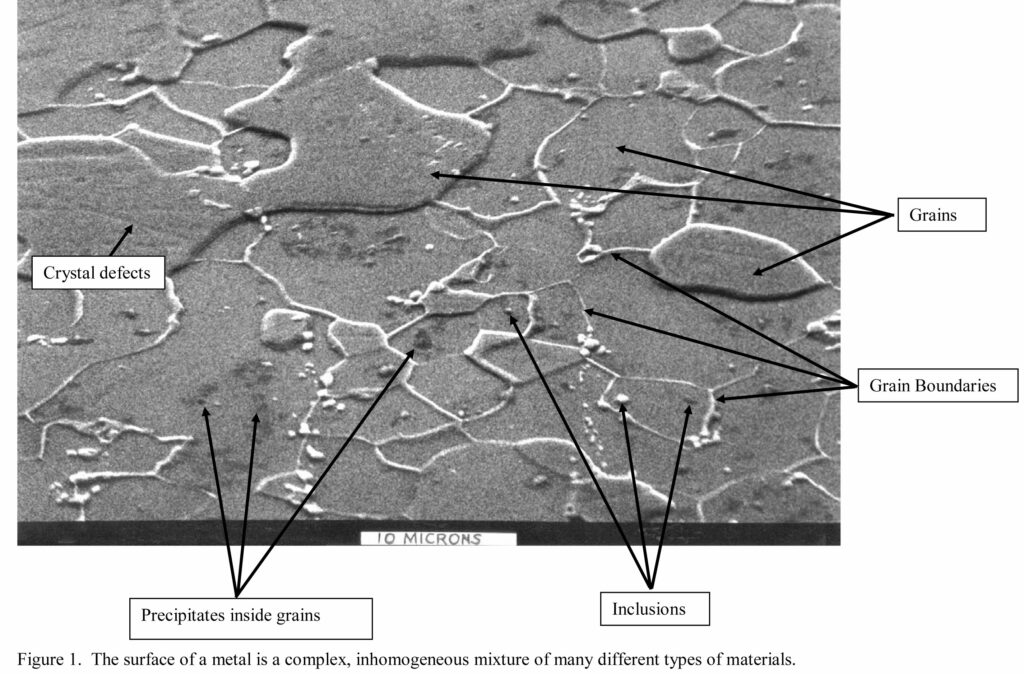
In summary, the surface of a metal container consists of a) a variety of different metal grains, b) different types of grain boundaries, c) inclusions, and d) defects in the grains. The distribution of each grain, grain boundary, precipitate, inclusion and defect is random, thus the exposure of each type of material to your formula is also random. This randomness contributes in part to the randomness of spray package corrosion.
The chemical composition of your formula determines which material on the package metal surface will or will not corrode. The chemical composition of your formula also determines the strength of bonds between the metal and a coating or a laminate film and the distribution of strong and weak bonds over the spray package metal surface.
Please send your questions/comments/suggestions to rustdr@pairodocspro.com.
Back issues of Corrosion Corner are available on CD from ST&M. Thanks for your interest and I’ll see you in April. SPRAY