Written on: July 1, 2013 by W. Stephen Tait
Aluminum spray package metals, internal coatings and laminate films are far from perfect (I will hereafter refer to coatings and laminate films as polymers). Indeed, the polymers are not always continuous (i.e., they have holes) and the metal surface is a complex mixture of different crystal structures and non-metallic materials.
In addition, a variety of aluminum alloys are used to fabricate aluminum spray packages. The different alloys often have different surface morphologies and thus polymers form different types of bonds with each type of morphology. The wide variety of polymer-metal bonds produces opportunities for spray package metal and polymer corrosion.
Anomalies on metal surfaces and anomalies in polymers could theoretically cause package corrosion. However, corrosion does not always occur when metal or polymer anomalies are present.
Let’s take a look at a few examples of the spray package metal and polymer anomalies, and discuss their relationship to spray package corrosion.
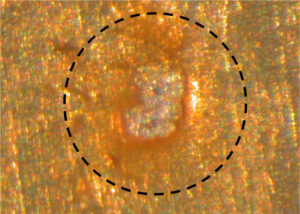 Figure 1. Hole in a polyacrylamide (PAM) coating
Figure 1. Hole in a polyacrylamide (PAM) coating
The anomaly in Figure 1 is a hole commonly found in internal coatings for aluminum aerosol containers. The hole directly exposes the aluminum metal to your formula.
Corrosion in aluminum aerosol containers does not occur at the same frequency as the holes. Consequently, holes in coatings do not necessarily lead to container corrosion.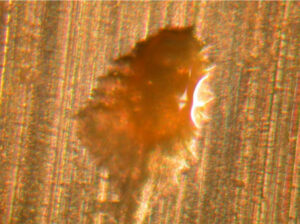 Figure 2. Divot in aluminum filled with coating (~0.2 mm wide along the minor axis)
Figure 2. Divot in aluminum filled with coating (~0.2 mm wide along the minor axis)
The anomaly in Figure 2 is an example of a divot on the metal surface that was created when a small piece of aluminum metal was torn from the metal during container fabrication. The coating flowed into the hole during coating application inside the container. Corrosion with this type of anomaly is rare.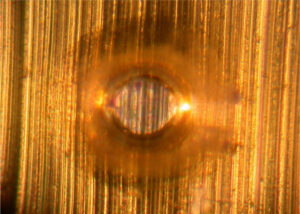
Figure 3. Solvent pop
Figure 3 has an example of a solvent pop. Solvent pops are very common with lacquer coatings, such as polyacrylamide (PAM). Small bubbles are created during heating to remove the solvent, and the bubbles produce solvent pops like that in Figure 3. Corrosion caused by solvent pops is also rare. Figure 4. Coating drool
Figure 4. Coating drool
The anomaly in Figure 4 is referred to as a drool. Drools form toward container bottoms when coating material drips from the spray nozzle used to apply the coating to the container. I’ve not observed instances where drools caused or contributed to corrosion.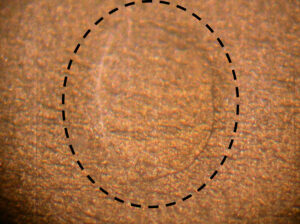
Figure 5. Nip in a laminated aluminum foil package (~0.3 mm wide along the minor axis)
The anomaly in Figure 5 is on a laminated aluminum foil bag inserted into traditional metal aerosol containers. This type of anomaly is referred to as a nip. Nips are common, but have not yet been shown to cause or contribute to bag metal or polymer corrosion.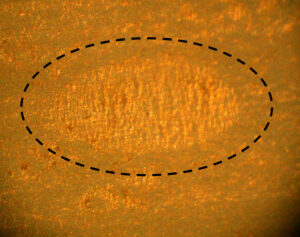
Figure 6. Distorted inclusion in aluminum metal (~0.65 mm wide along the minor axis)
The anomaly in Figure 6 was formed from an inclusion in the aluminum metal. Inclusions are alloying materials in aluminum alloys that precipitate when the molten aluminum alloy is cooled to room temperature.
The inclusion in Figure 6 was originally a spherical particle that was distorted into an oval during container fabrication. This type of anomaly could cause container pitting corrosion. 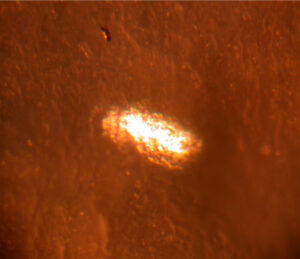 Figure 7. Crack in aluminum foil used for laminated foil bags
Figure 7. Crack in aluminum foil used for laminated foil bags
Figure 7 has an example of a crack in the aluminum foil between layers of polymer films. This type of laminate is used to fabricate the internal bags inserted into traditional aerosol containers.
Cracks in the aluminum foil are common. Product leakage could occur through a crack whenever product diffuses through a laminate film.
The examples shown in this month’s Corrosion Corner are a few of those used in our Elements of Spray Package (Aerosol) Corrosion short course. This 1.5 day short course provides an introduction to all aspects of spray package corrosion, corrosion testing and corrosion prevention. More info: www.pairodocspro.com.
Please send your questions/comments/suggestions to rustdr@pairodocspro.com. Back issues of Corrosion Corner are available on CD from ST&M. Thanks for your interest and I’ll see you in August. SPRAY