How does concentration affect spray package corrosion? Part 1
Written on: January 18, 2016 by W. Stephen Tait
Happy New Year, everyone. The chemical composition of a formula determines if corrosion will occur, where it will occur and how fast corrosion proceeds through the package metal to cause leaking or how fast the package polymer degrades. The concentrations of formula ingredients may also affect spray package corrosion.
A list of the most likely formula ingredients that could contribute to or cause spray package corrosion is:
- pH
- Water
- Corrosion inhibitors
- Fragrance
- Surfactants
- Electrochemically active formula ingredients, copper ions, chloride ions, nitrate and sulfate ions
- Propellants
I will discuss the first four items this month, and the remainder next month.
pH: (hydrogen ion concentration)
The concentration of hydrogen ions in the formula or contaminant water is quantified with pH—the negative logarithm of the hydrogen ion concentration in water. Therefore a pH of two is 10-2 moles per liter of hydrogen ions (acidic) and a pH of 12 is 10-12 hydrogen ions (basic).
The shape of a pH-corrosion rate curve for individual formula-package systems is dependent on the chemical composition of your formula and the type of package metal. Figure 1 is an example of how
water pH affects the corrosion rate of iron.
The Y-axis is the corrosion rate in mils per year (1 mil = 0.001 inches) and the X-axis is the pH. Notice that the corrosion rate is very high at a pH of three (approximately 580 mils per year), and increasing
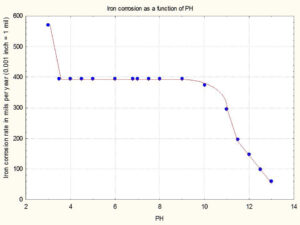
Figure 1. Example of how pH could affect steel corrosion rates (Click Image to Enlarge)
the pH to 13 decreases the corrosion rate to approximately 50 mils per year.
A normal rated tinplated steel container has a nominal thickness of six mils. Therefore, if Figure 1 is valid for your container and formula, the container service life is approximately 44 days when the pH is 13.
Water (either as a formula ingredient or as a contaminant)
Water is electrochemically active and therefore could cause spray package metal corrosion. Water also absorbs into polymer coatings and laminate films, causing their physical properties to degrade, such as with the polymer glass transition temperature. Polymers lose their physical properties when the temperature is above the glass transition temperature.
Liquid water is necessary for metal and polymer corrosion. The amount of water molecules needed to form liquid water depends on the chemical composition of your formula. However, approximately 90 water molecules thermodynamically constitute pure liquid water.
Water is consumed during metal and polymer corrosion and must be replenished to maintain the progress of the corrosion. For example, it would take approximately 0.042 ppm of water in 100 grams of formula to sustain pit growth until it perforated a 6-mil thick container.
There have been many instances where spray package corrosion does not occur when the water concentration range is above and below a critical concentration. Therefore, the critical concentration for package corrosion by water is determined by the chemical composition of the liquid (formula + propellant + water) in the commercially filled spray package.
There are no public domain lists of critical water concentrations for spray formulas, so the non-corrosive concentration of water in specific formulas should be determined on a case-by-case basis.
Corrosion inhibitors
Corrosion inhibitors typically have an effective concentration range that prevents or controls corrosion. Corrosion typically is higher when inhibitor concentrations are both below and above the effective concentration range.
Figure 2 provides an example from the Pair O Docs corrosion inhibitor research program. It was generated with unlined tinplated steel exposed to inhibited water at a pH of seven.
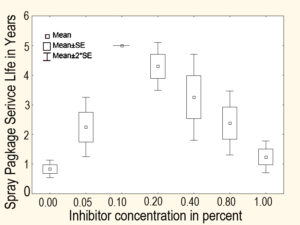
Figure 2: An example of how corrosion inhibitor concentration could affect spray package service lifetime. (Click Image to Enlarge)
The Y-axis is the spray package service lifetime estimated from electrochemical corrosion measurements and the X-axis is the inhibitor concentration. Notice that the maximum package service lifetime occurs at a 0.1% concentration. Notice also that the effective inhibitor concentration range is very narrow—spray package service life decreases when the inhibitor concentration is above and below 0.1%.
There are no public domain lists of the effective corrosion inhibitor concentrations for spray formulas, so the effective concentration range in specific formulas should be determined on a case-by-case basis.
Fragrance
There are a small number of fragrance types that contribute to or cause spray package corrosion. However, most fragrances typically act as corrosion inhibitors. Indeed, there are numerous examples of natural ingredients that act as corrosion inhibitors with specific environments (i.e., your formula).
Fragrances also have an effective concentration range for inhibiting corrosion—much like there is a fragrance concentration in your formula that gives it the optimum performance. There is no public domain date for corrosion rates as a function of fragrance concentration, so the non-corrosive concentration range for individual fragrances needs to also be determined on a case-by-case basis for specific formulas.
Next month’s Corrosion Corner will feature Part 2 of the discussion on how formula ingredient concentrations affect spray package corrosion by:
- Surfactants
- Electrochemically active formula ingredients, copper ions, chloride ions, nitrate and sulfate ions
- Propellants
We would be happy to teach our Elements of Spray Package (Aerosol Container) Corrosion short course at your R&D facility. Want a specific topic discussed in an issue of Corrosion Corner? Please send your suggestion/questions/comments to rustdr@pairodocspro.com or visit www.pairodocspro.com. Back articles of Corrosion Corner are available from Spray. Thanks for your interest and I’ll see you in February. Spray
