Written on: April 1, 2018 by W. Stephen Tait
Hello, everyone. Last month, we discussed the first three of six common questions about spray package corrosion; we’ll tackle the final three questions this month.

Anhydrous formulas are immune from corrosion—true or false?
Kind of True
A completely anhydrous formula is not expected to corrode spray packages. However, does a completely anhydrous formula exist? For example, humid factory air introduces small amounts of water (parts per million) into a formula during manufacturing and a contaminated anhydrous formula could be a voracious package-eater.
Strangely, completely anhydrous ethanol aggressively corrodes aluminum, but a few parts per million of water in ethanol prevents aluminum corrosion. In other words, there are no simple guidelines for when and how much contaminant water will or will not cause spray package corrosion.
You can always rely on sodium nitrite to inhibit corrosion—true or false?
Cautiously true
Sodium nitrite inhibits a wide range of corrosive liquids but it doesn’t work for all formulas. Indeed, no corrosion inhibitor does.
Sodium nitrite is consumed while inhibiting corrosion. It also oxidizes formula ingredients, causing the formula to yellow. Sodium nitrite consumption depletes its concentration and thus sodium nitrite is often referred to as an unreliable inhibitor. Figure 1 illustrates why it’s considered unreliable.
Notice the corrosion rate in Figure 1 is approximately zero at a concentration around 0.1% (for this specific metal-formula system). Notice also that the corrosion rate increases when the concentration of nitrite is below 0.1% and above 0.1%. In other words, too little or too much sodium nitrite actually causes corrosion instead of inhibiting it.
Figure 1: Corrosion rate as a function of sodium nitrite concentration
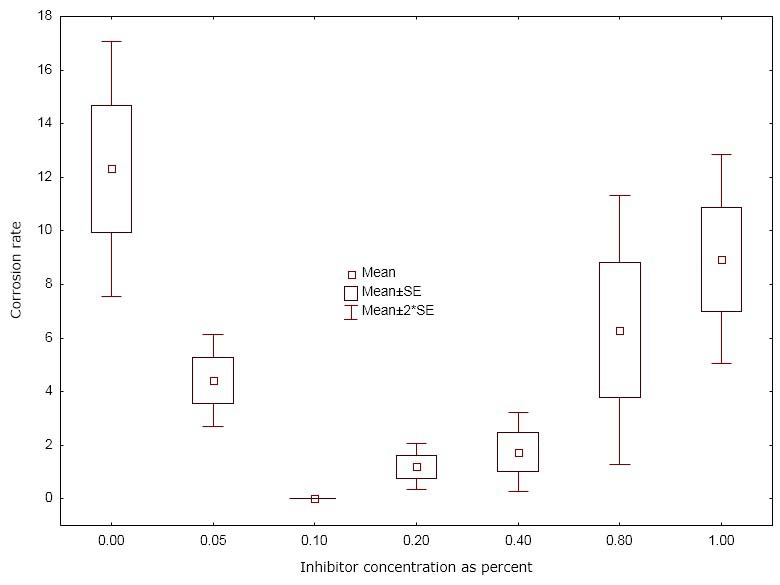
Therefore, a sodium nitrite inhibited formula may initially be non-corrosive but could become very corrosive as the formula-package system ages and the nitrite is consumed. Sodium nitrite could also form nitrosamines or nitrous oxide gas in low pH formulas.
Corrosion can concentrate in holes in polymer coatings and thus cause pitting corrosion—true or false?
Emphatically False!
Holes in coatings that expose the package metal are very common. In one instance, I found approximately 25% of the test containers had at least one very small hole in the internal polymer coating.
Figure 2 provides examples of holes in a polymer coating on aluminum and tin coating on tinplated steel. The lighter colored “circle” in the center of the tinplate example is detinning and the edge of the hole is next to the dashed oval.


Coating holes like those in Figure 2 typically occur because metal surfaces are complex and random mixtures of crystal defects, pure metals, metal alloys, different types of metal crystals, grain boundaries and non-metal particles called inclusions. Polymer and tin coatings will not bond with many of these defects and surface materials, causing non-wetted areas that become a hole in the coating like those in Figure 2. SPRAY