Written on: July 1, 2018 by W. Stephen Tait
Hello, everyone. It is often assumed that a higher storage temperature will increase the rates of polymer and metal corrosion and thus reduce the time needed for corrosion testing. For example, it’s common to assume that 3–6 months of testing at a high storage temperature will produce corrosion that would be observed after several years of testing at room temperature. However, routine use of high temperatures to shorten storage test times often leads to unexpected corrosion.
Chemical reaction rates double for every 10°C increase in temperature when the reaction has first order kinetics that are controlled by the activation energy of the chemical reaction. However, the corrosion rates of spray package materials do not follow the rate-temperature relationship as illustrated in Figure 1. The corrosion rate on the Y-axis in Figure 1 is in millimeters per year of metal corrosion under an epoxy coating and the corresponding measurement temperature is plotted on the X-axis in °C.
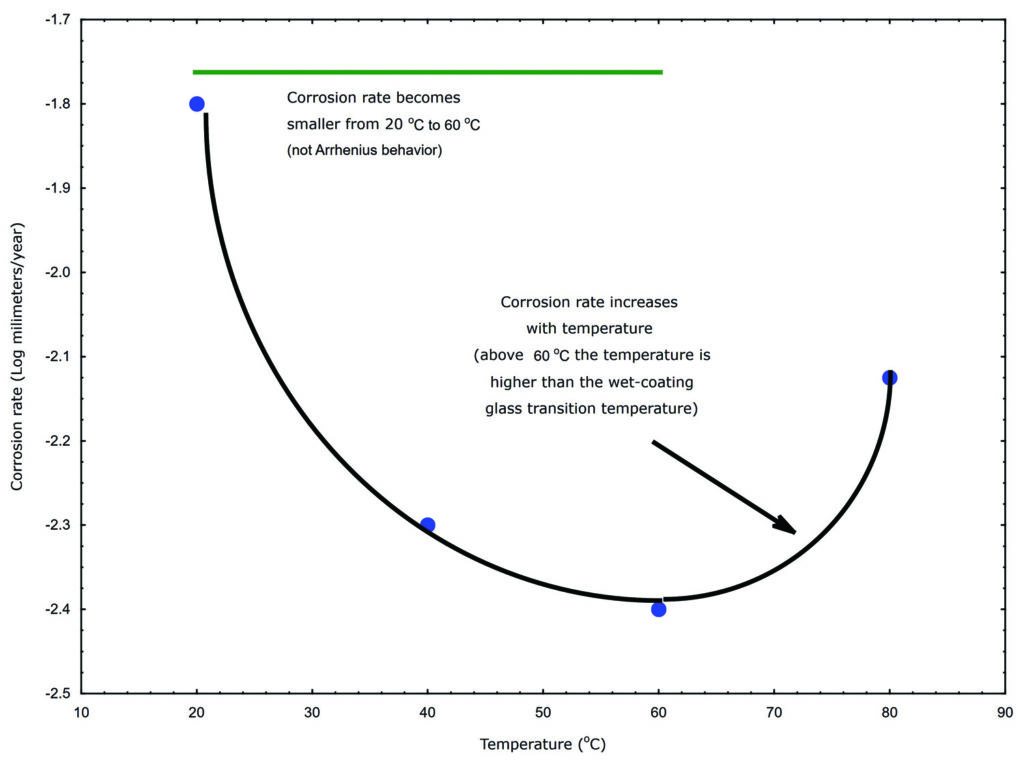
Figure 1: coated metal corrosion rate-temperature trends
Notice that as the temperatures increases from approximately 20°C to 30°C, the corrosion rate decreases from approximately 0.016mm per year to 0.006mm per year (10-1.8 to 10-2.2). In other words, increasing the temperature from 20°C to 30°C decreased the corrosion rate instead of doubling the rate. Notice also that the corrosion rate suddenly increases above 60°C.
Polymer properties degrade at a threshold temperature referred to as the glass transition temperature (Tg). Polymers lose physical properties above their Tg, such as being a barrier between the underlying package metal and your formula. In other words, the polymer is not a barrier between the package metal and your formula when the temperature is above Tg.
The corrosion rate increase around 60°C in Figure 1 suggests that the Tg for the polymer is around 60°C. The polymer in this example is an epoxy and a dry epoxy typically has a 100°C Tg. Thus, Figure 1 also illustrates that a wet polymer has a lower Tg than the corresponding dry polymer.
Metal and polymer corrosion rates are not first order kinetics and their kinetics typically cannot be modeled with pseudo first order reactions. In addition, metal corrosion is not a pure chemical reaction. Metal corrosion is instead a change of the metal’s chemical state that occurs when metal valence electrons are removed from surface metal atoms by electrochemically active ions and/or molecules. Metal corrosion rates are also not solely determined by the activation energy.
Consequently, metal corrosion rates do not double for each 10°C storage test temperature increase, thus higher storage temperatures do not accelerate metal corrosion rates. It is also common to observe aerosol containers:
Please note that conducting tests at higher temperatures is a necessary part of qualifying a package with a formula. Higher temperature testing is needed to determine formula thermal stability, particularly when your products are marketed in regions with high year-round or summer temperatures.
Higher storage temperatures are used to determine if products are thermally stable and if thermal instability leads to corrosion of package materials. However, it is recommended to:
Is it possible to reduce corrosion test time?
Attempting to accelerate package material corrosion with higher temperatures or other means is not recommended. However, the time needed for spray package corrosion testing can be reduced by measuring corrosion with sensitive electrochemical corrosion instruments.
Sensitive instruments measure polymer and metal corrosion long before either can be seen with the unaided eye. However, the appropriate measurement parameters, exposure times, analysis protocols, sample size and protocols for interpreting the corrosion data are all needed to obtain reliable results with electrochemical corrosion tests.
Thanks for your interest and I’ll see you in August. SPRAY