Written on: February 1, 2019 by W. Stephen Tait
Hello, everyone. Last month I discussed a first-principles empirical equation for predicting if corrosion could occur. However, the equation only indicates if corrosion might occur and provides no information on how fast corrosion will occur and the type or types of package corrosion that are expected.
The corrosion rate through spray package materials determines the package service lifetime—the length of time before a spray package leaks. Currently there are nine known factors that influence the magnitude of spray package corrosion rates:
These factors could contribute to or cause corrosion either independently or synergistically with one or more of the other factors.
One possible empirical equation for predicting spray package corrosion rates is listed below. Temperature is not included in this equation because increasing temperature typically does not accelerate spray package corrosion rates.
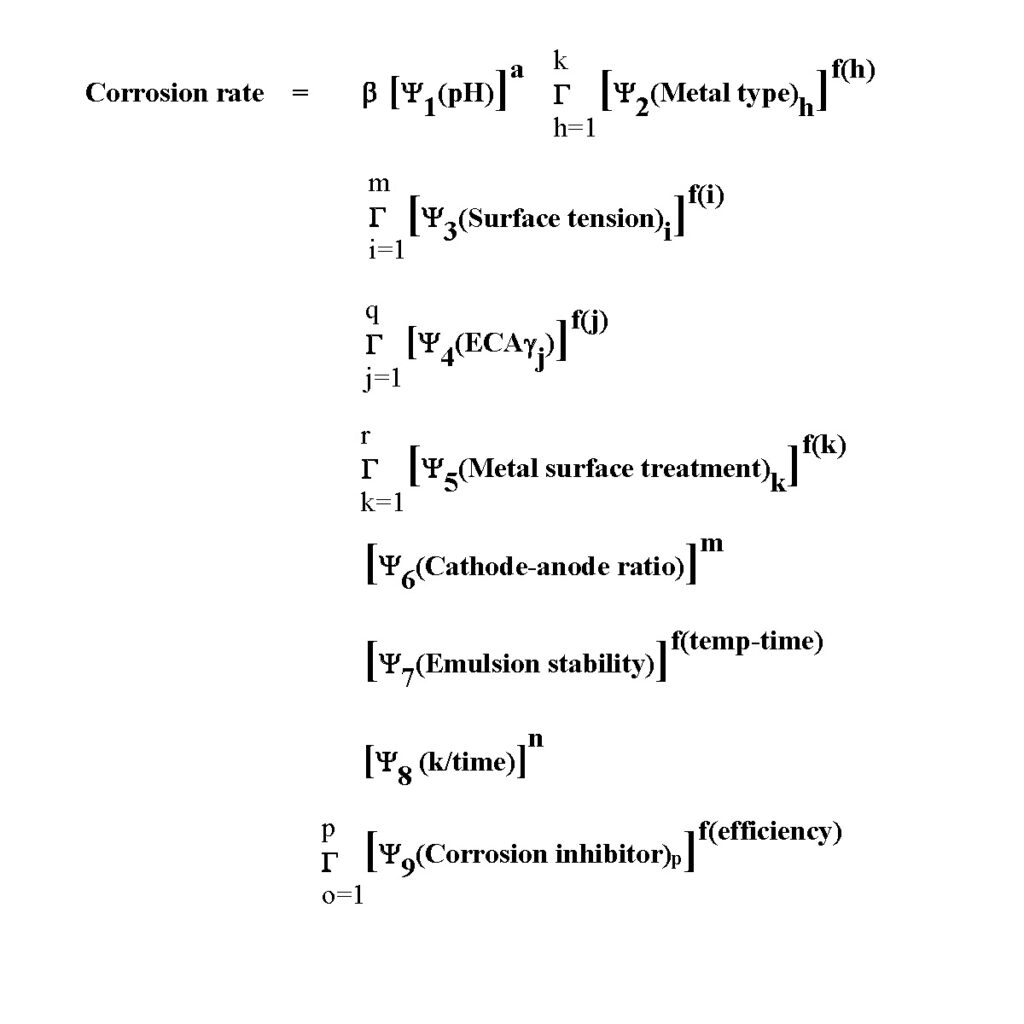
The first symbol in the Equation, b, is a conversion constant. The corrosion penetration rate could be inches/year or millimeters/per year, depending on the units for b.
The Gsymbol indicates that a factor is the multiplication of multiple components. In addition, all none factors are multiplied together instead of added to account for synergy between one or more factors.
The letters equal to one (e.g., h = 1) under the Gsymbol and the letters above the Gsymbol indicate that there are ranges of components for each factor. For example, the surface tension factor for a formula with three surface-active ingredients is equal to the multiplication of the probabilities for each surface-active ingredient—or stated in the equation form:
Surface tension factor = Ψ4 (surface tension1)f(1) times Ψ4(surface tension2)f(2) times Ψ4(surface tension3)f(3)
Notice that each of the nine factors has multiple components. Let’s briefly discuss the significance of each factor and its components.
Factor 1

The first factor estimates how pH affects the corrosion rate magnitude. The symbol Y1 is the probability that formula water or contaminant water pH will decrease or increase the corrosion rate and the exponent “a” determines how much pH affects the rate. The exponent is typically a single number.
Factor 2

Spray package metals could be tinplated steel, tin-free-steel, aluminum and aluminum foils. Both coated/laminated metals and uncoated metals corrode at different rates when exposed to the same formula. Consequently, the second factor accounts for how different types of metals influence the rate of spray package corrosion. The exponent f(h) is a complex function that generates a single number for each metal type exposed to a specific formula.
Factor 3

The formula-spray package surface tension determines how effectively various formula ingredients wet the internal package materials. Wetting determines how easy or difficult it is for formula ingredients to absorb into and diffuse through polymer coatings and laminate films and onto uncoated or substrate metals under laminated films and coatings. The exponent f(i) is a complex function that generates a single number for each component surface tension.
Factor 4

The forth factor accounts for how electrochemically active (ECA) ions and molecules affect corrosion rates. The ECAgj symbol represents the electrochemical activity for individual ions and molecules. The exponent f(j) is a complex function that generates a single number for each specific electrochemically active ion or molecule in a formula.
Factor 5

The fifth factor accounts for how the metal substrate surface treatment affects the corrosion rate. Surface treatments include polymer coatings or laminate films, tin coatings on steel and chromium/chromium oxide coatings on steel. Note that tin coatings are typically a complex multilayer surface treatment. The exponent is a complex function that generates a single number for each type of surface treatment.
Factor 6

Metal surfaces—both coated and uncoated—are composed of cathodic areas where valence electrons are transferred from surface atoms to electrochemically active (ECA) formula ingredients and anodic areas where the atoms are ejected from the bulk metal as ions. The cathode/anode ratio determines pitting corrosion rates. The exponent f(m) is a function whose value is determined by the specific chemical composition of a formula and the type of package materials.
Factor 7

Emulsions break after a certain time (or age) and can also be broken with either high or low temperatures. Water and cream phases are typically generated when an emulsion breaks, and one or more of these phases could be very corrosive. Consequently, the exponent for this factor is an equation that is a function of temperature and emulsion age. This particular factor is zero for non-emulsion products. In other words, this factor is equal to one for non-emulsion products.
Factor 8

Steady state corrosion rates are used to estimate spray package service lifetime. Steady state rates are achieved after spray packages are filled and the time-to-steady-state could range from a few days to nine months after filling. The exponent “n” for this factor is a single number that is determined by the specific chemical composition of a formula and the type of package materials.
For example, an uncoated tinplated container might rapidly de-tin (tin coating corrosion) and the large number of tin ions quickly break the emulsion. Conversely, a coated tinplate container with the same formula would also most likely detin, but the number of tin ions generated would be lower and the emulsion would probably break more slowly.
Factor 9

There is no such thing as a one-size-fits-all corrosion inhibitor. There are also many types of formula ingredients, such as fragrances, that in some instances act as corrosion inhibitors. Consequently, the exponent of this factor is a complex function that accounts for specific formula chemical compositions, pH, synergy between all ingredients that have the ability to inhibit corrosion and the effective concentration range for each ingredient that can inhibit corrosion.
The empirical corrosion rate equation presented in this Corrosion Corner could be used to estimate package service lifetime when every probability is known for a specific formula chemical composition. However—as with last month’s equation—most of the probabilities for each factor and individual factor-components are unknown. In addition, the probabilities and factor components are unique to each formula chemical composition and type of package for the formula.
The majority of the components for each factor in the equation are either unknown or not available in the public domain at this time. Consequently, corrosion testing is the only reliable way to determine:
We plan to drive from Wisconsin to California and back for two weeks from late April–early May 2019. Consequently, Pair O Docs would be pleased to teach our Elements of Spray Package Corrosion short course at your company if you are located west of Wisconsin (north or south route). Please contact me at 608-831-2076 or rustdr@pairodocspro.com if interested. Thanks for your interest and I’ll see you March. SPRAY