Written on: November 1, 2017 by W. Stephen Tait
Hello, everyone. Last month we had Part II of a five-part series on the metals used to fabricate spray packaging. This month, we continue with a discussion of tin-free-steel, more commonly referred to as TFS.
Sheets of TFS have a similar chemical composition to that for the steel used to make ETP (tinplated steel). However, the surface treatment for TFS is much simpler, as shown by the diagram in Figure 1. Notice that the surface of TFS is essentially only one layer of a very thin mixture of metallic chromium and chromium oxide.
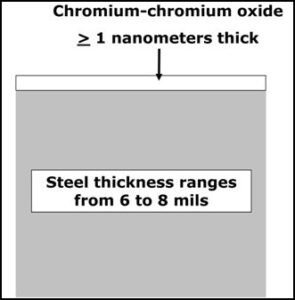
Figure 1: Tin free steel structure
TFS is commonly used to fabricate food containers, but there are steel aerosol containers that are also fabricated with TFS.
There is an old axiom that says polymer coatings bond more strongly to chromium than to tin. However, I’ve not found any referenced technical publications that either prove or disprove this saying.
The corrosion rate-pH behavior for both TFS and ETP are determined by the base steel and chemical composition of a formula. Figure 2 provides a corrosion rate graph for steel as a function of pH magnitude. This graph is a very old one and was generated for a steel alloy similar to that used for aerosol containers. The solution for this graph is water.
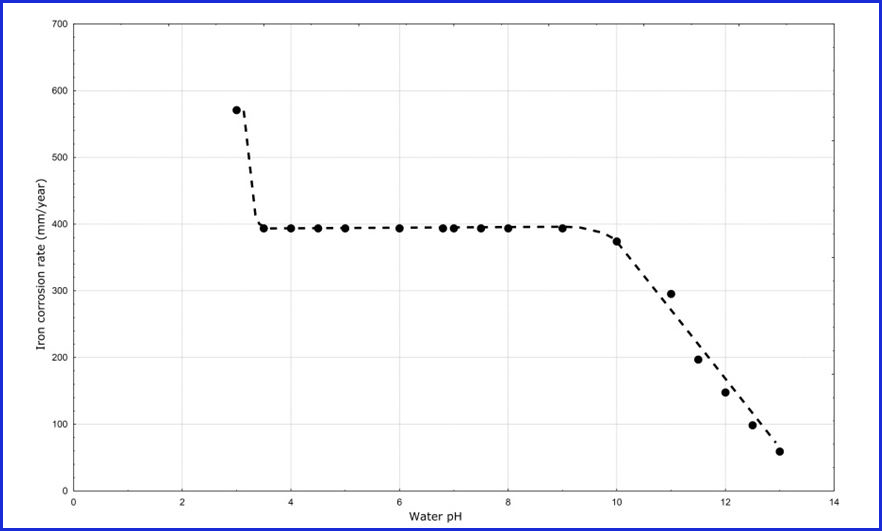
Figure 2: Steel corrosion rates as a function of solution pH
The interpretation of the graph in Figure 2 is complex and often leads to the incorrect conclusion that corrosion is prevented by high pH. Indeed, the curve in Figure 1 is the basis for the corrosion myth that increasing pH stops TFS and ETP aerosol container corrosion.
For example, notice in Figure 2 that when the pH is 13 the corrosion rate is approximately 50 millimeters per year—a very high corrosion rate. The service lifetime for a 2Q aerosol container with this high corrosion rate would be approximately two days. In other words, even though increasing pH does decrease the corrosion rate, it might not decrease the rate to a low enough magnitude to result in a commercially acceptable container service life.
Other ingredients in a formula could change the actual pH range that produces low corrosion, produce significantly higher or lower corrosion rates from those in Figure 2 at any given pH, and change the shape of the pH-corrosion rate curve altogether. In other words, Figure 2 only illustrates that steel corrosion rates are a complex function of pH
Consequently, changing a formula’s pH should be accompanied with corrosion tests to determine if the pH-change will increase or decrease TFS container and ETP container steel corrosion.
Next month we’ll continue the series with a discussion of stainless steel.
Pair O Docs has a state-of-the art electrochemical corrosion testing laboratory; please contact me if you would like to know more about our faster and predictive corrosion testing. You can also visit our new website which has a short Vision Video that discusses all our corrosion prevention and control services. Please also contact me if you would like to a have our Elements of Spray Package Corrosion short course taught at your R&D facility. Back issues of Corrosion Corner are available on CD-Rom. Thanks for reading and I’ll see you in December. SPRAY