Written on: September 8, 2021 by Cassandra Taylor
The global regulatory landscape is constantly evolving to meet the needs of different groups, such as industry, workers and consumers. The needs of different groups, such as industry, workers and consumers. As new health and safety information becomes available, countries must adapt to ensure that product users and producers alike are given the tools and information required to use hazardous chemicals safely and effectively. This column will provide an overview of recent key regulatory updates in several Asian jurisdictions.
China introduces exemption for imported cosmetic animal testing
On March 4, 2021, China’s National Medical Products Administration (NMPA) released the finalized Provisions for Management  of Cosmetic Registration & Notification Dossiers. Effective May 1, 2021, “general” cosmetic products can now be imported into China without having to first be tested on animals. Chinese regulations distinguish between “special-use” and “general” cosmetics. Special-use cosmetics include products such as hair dyes, hair perming products, sunscreens and cosmetics “claiming new efficacy.” All other products complying with the definition of cosmetics given by the Cosmetic Supervision & Administration Regulation (CSAR) are classified as “general” cosmetics.
of Cosmetic Registration & Notification Dossiers. Effective May 1, 2021, “general” cosmetic products can now be imported into China without having to first be tested on animals. Chinese regulations distinguish between “special-use” and “general” cosmetics. Special-use cosmetics include products such as hair dyes, hair perming products, sunscreens and cosmetics “claiming new efficacy.” All other products complying with the definition of cosmetics given by the Cosmetic Supervision & Administration Regulation (CSAR) are classified as “general” cosmetics.
According to the final rule, two documents must be provided in order to benefit from the exemption:
1. A good manufacturing practices (GMP) certificate of compliance issued by a competent authority of the country of origin; and

2. Results of the product’s safety assessment, confirming that the products are safe to use.
In addition to the above requirements, there are a few exceptions when general cosmetics will still require testing on animals for import into China:
• Products that claim they are intended to be used on children/infants;
• Products using new cosmetic ingredients (as defined by the Chinese regulations) during the compulsory three-year monitoring period; and
• Instances where the notifier, importer or manufacturer is listed as a key supervision target according to the results of the quantitative rating system established by the NMPA.
Although there are still some restrictions, this is a good step forward in terms of increasing the accessibility of cruelty-free products in the Chinese market.
South Korea looks to expand definition of existing substances under K-REACH
Earlier this year, South Korea’s Ministry of Environment consulted on a plan to expand the definition of existing substances under K-REACH. 
The Act on Registration & Evaluation of Chemicals (K-REACH, also known as ARECs) regulates the designation of hazardous chemical substances in South Korea through registration and evaluation. K-REACH mandates that new chemical substances be registered or notified before production or import. Existing chemical substances need to be registered when the production or import volume exceeds one ton/year.
Existing substances are currently defined under K-REACH as one of the following:
• Chemical substances publicly notified by the Minister of Environment in consultation with the Minister of Employment & Labor, which were domestically distributed for commercial purposes in South Korea prior to Feb. 2, 1991; or
• Chemical substances publicly notified by the Minister of Environment for which, after Feb. 2, 1991, a hazard assessment has been conducted pursuant to the former Toxic Chemicals Control Act.
The draft amendment, which was published on March 2, 2021, adds three new criteria to the definition of existing substances:
1. Isomers of an existing substance;
2. Hydrates or anhydrides of an existing substance; and
3. Reaction products consisting of two or more existing substances.
In cases where an existing chemical substance was notified as a specific isomer, the new definition excludes isomers of the already-notified substance. This means new isomers of an existing substance will still need to be notified if the original substance was a specific isomer rather than mixture of isomers or unspecified. Additionally, reaction products would only be considered “existing” in cases where separation of individual components is technically difficult and therefore the product is distributed on the market as a mixture.
There are currently more than 43,000 substances on the Korea Existing Chemicals List (KECL). This amendment would further expand the group of chemicals considered for listing and therefore distribution in South Korea.
Japan sets preliminary schedule for mandatory SDS, labels
Japan’s Ministry of Health, Labor & Welfare (MHLW) is planning to expand the number of hazardous chemicals that are required to have Safety Data Sheets (SDS) and labels under its  Industrial Health & Safety Law (ISHL).
Industrial Health & Safety Law (ISHL).
The ISHL requires suppliers to prepare an SDS and label for substances listed in Table 9 of the cabinet order of the ISHL, which currently identifies 633 substances. An SDS and label must also be supplied for mixtures containing designated substances above the applicable cut-offs found in Table 2 of the Ordinance of ISHL.
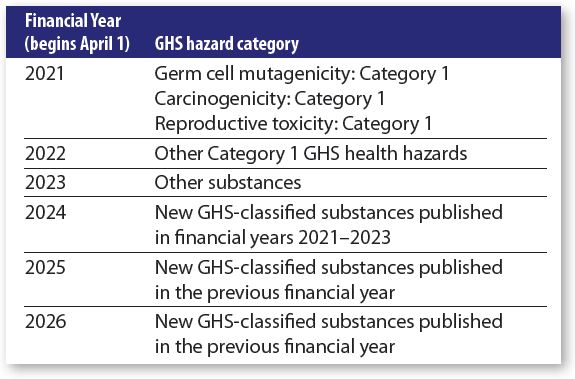
On Feb. 26, 2021, MHLW published a preliminary five-year schedule outlining the plan to add several hundred new substances to Table 9 each year for the next three years.
According to the interim report from MHLW’s twelfth management of chemical substances in the workplace study group, approximately 700 new substances per year will be added for the first three years. This is followed by a transitional period of two to three years for the new substances.
Additional substances to be listed in Table 9 will be prioritized according to the Globally Harmonized System of Classification & Labeling of Chemicals (GHS) categories assigned by the MHLW, Ministry of Economy, Trade & Industry (Meti) and the Ministry of Environment (MoE). The preliminary schedule appears in the chart at bottom left.
As the table shows, substances classified as GHS Category 1 carcinogens, mutagens and reproductive toxicants (CMRs) will be prioritized for listing this year. These will then be followed by substances with other Category 1 GHS health hazards in 2022 and other hazardous substances in 2023.
Japan GHS chemical classifications are published on the National Institute of Technology & Evaluation (NITE) website as a reference for companies preparing hazard communication documentation. In April, NITE introduced the GHS Mixture Classification & Labeling Creation System (NITE-Gmiccs) web tool to help companies search for GHS classifications and create labels.
Thailand to publish 5th revision of Hazardous Substance Act
Thailand’s Ministry of Industry (MoI) has approved a fifth  revision of the Hazardous
revision of the Hazardous
Substances Act, the country’s main chemical control law. The updated regulation, which is anticipated to be published sometime this year, will expand the hazardous substance notification requirements in Thailand.
The new revision is expected to include draft amendments to notification on the declaration of List 5.6 hazardous substances and notification for R&D exemption BE 25XX.
Under List 5.6, the reporting requirements will shift from focus on a product to a single substance. Currently, companies must notify the Dept. of Industrial Works (DIW) if the total accumulated quantity of hazardous substances in all products they manufacture or import exceeds one (1) ton per year. This will change to a requirement to notify if the total amount of a single substance or single mixture exceeds one (1) ton.
The amended notification for R&D exemption will replace the existing MoI Notification on R&D Exemption BE 2559. Anticipated changes under BE 25XX include the following:
• Expansion to include education, testing and analysis;
• Notification and registration for Type 2 (flammable) substances will not be required;
• Licensing and registration for Type 3 (oxidizing agent or peroxide) substances will not be required;
• Substances will need to comply with UN GHS; and
• Exemptions will be valid for six months and are non-renewable.
We will be sure to keep you in the loop as new hazard communication requirements are implemented in Asia and around the world. SPRAY