Written on: October 1, 2023 by W. Stephen Tait
Hello, everyone. It’s commonly believed that a barrier is needed between a spray package’s base metal/metal foil and a formula to prevent corrosion of the metal/metal foil packaging. It’s also commonly believed that coatings and laminate films are these barriers. I’ll refer to both coatings and laminate films as “coatings.”
Coatings do not always prevent corrosion and are not always needed to prevent corrosion—Why? This month starts a three-part series discussing why coatings are not always corrosion barriers for spray packages.
Why coatings are not perfect barriers
Metal surfaces are a complex mixture of:
• A variety of metal/metal alloy crystal groups, called grains
• Thin, non-metallic intergranular boundaries between different grains—the glue keeping them together
• A variety of metal-alloy/non-metallic precipitate particles inside grains
• Metal structural defects inside grains
Consequently, complete coating coverage over a package’s metal surface requires that a coating bonds to all the metal surface attributes listed above. Not surprisingly, the complexity of a metal surface prevents complete coating coverage, resulting in microscopic and macroscopic coating defects wherever coating/metal bonding is incomplete or missing.
Figure 1 provides an example of a macroscopic solvent pop in a coating. This solvent pop has an approximately 100-micron diameter.

Solvent pops are very common in both coated steel and coated aluminum packages. Indeed, in one instance, approximately 25% of the packages from the same manufacturing lot had coating solvent pops.
A thin cap over the solvent pop is the transparent area inside the dark halo ring. Caps are typically removed/dissolved shortly after a package is filled. However, metal pitting corrosion in solvent pops only initiate and are sustained when a large area of the coating surrounding the solvent pop is also corroding.
Figure 2 provides an example of a microscopic hole in a coating where it did not wet the substrate metal. The hole diameter is approximately 20-microns—similar to that of human hair—and it exposes the metal to a formula. Coating holes are commonly found in both coated steel and coated aluminum packages.
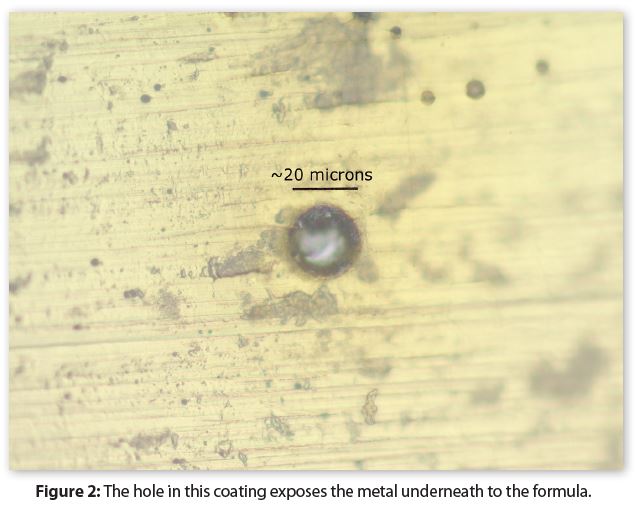
It’s tempting to conclude that a hole in a coating causes metal pitting corrosion. However, in a coated metal, pitting corrosion initiates, and is sustained, only when a large area of the coating surrounding the hole is also corroding, much like that for the solvent pop in Figure 1.
Coatings also have other microscopic defects that allow liquids to absorb into, and accumulate in, a coating. Absorbed liquid often saturates a coating, breaks down coating barrier properties and causes metal corrosion under the coating (Absorbed liquid diffuses into and through a coating; Adsorbed liquid sits on a coating surface).
The two most common microscopic coating defects are pores and voids, which occur in all coatings, are very small and difficult to observe. Thus, coating pores and voids are typically indirectly detected with analytical methods, such as positron annihilation spectroscopy and electrochemical measurements.
Liquid (from a formula) moving into and through pores often enlarge pores and create microscopic rivers. These rivers often cause extensive coating corrosion (e.g., blistering), loss of the coating’s barrier properties and metal corrosion under the coating.
In some instances, swelling of a coating can slow or stop corrosion by pinching off rivers. Corrosion products can also slow or stop metal corrosion when metal corrosion products backflow into and plug a river.
Figure 3 has a diagram of a model for how liquids move into and through coating pores and voids. The blue lines depict pores full of liquid and the blue ovals depict voids full of liquid.
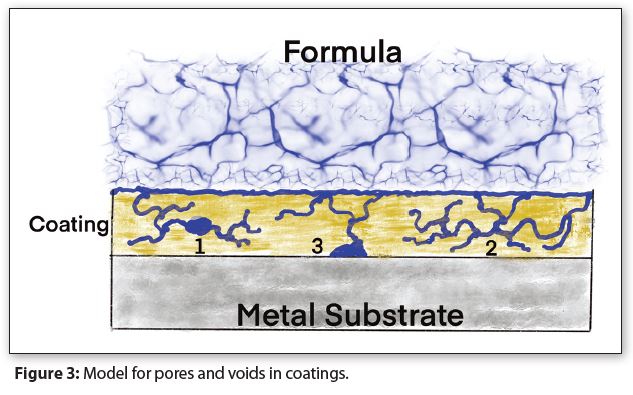
Liquid-filled pores form microscopic rivers in the coating that disperse liquid throughout the coating and deliver it to voids at the coating-metal interface. The chemical composition of the microscopic rivers could be either the same as, or significantly different from, a formula’s chemical composition. The numbers in Figure 3 indicate:
1. Pores end in voids: #1 depicts pores that end at a coating void. Capillary action fills both the pores and void with liquid.
2. Meandering pores: #2 depicts pores where a liquid meanders throughout the coating without ending at a void. Both pores ending at coating voids and meandering pores typically cause coating corrosion—such as swelling and blistering—that can also delaminate a coating from its substrate metal in addition to breaking down coating barrier properties.
3. Pores with Voids at the Metal-Coating Interface: #3 depicts pores that end at a void at the coating-metal interface. The liquid accumulating at the interface voids often leads to metal corrosion, as well as coating delamination, such as blisters.
Metal corrosion occurs under a coating when:
• The liquid moving through the coating is corrosive toward the metal
• Sufficient liquid accumulates at the metal-coating interface
• Liquid continuously flows through the microscopic rivers and the flow is large enough to sustain metal corrosion
• Coating swelling and corrosion products do not pinch or plug the microscopic rivers
In other words, when/if a coating will either protect, or not protect, the substrate metal from corrosion is determined by a very complex interaction between a formula’s chemical composition and the type of metal, type of coating, and metal/coating morphologies (grains/grain boundaries/precipitates/defects, coating pores and voids, respectively).
Pores and voids are always present in coatings and typically not visible to the unaided eye or a light microscope. Consequently, electrochemical measurements with sensitive instruments are needed to determine if these defects will or will not cause package corrosion with a given formula.
We’ll continue this three-part discussion in the next issue. Thanks for your interest and I’ll see you in November. Contact me at 608-831-2076; rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY