Written on: December 1, 2023 by W. Stephen Tait
Hello, everyone. This month, we’ll complete the three-part series begun in (part 1, October), (part 2, November) with a discussion on how coating glass transition temperature (Tg) changes when formula ingredients permeate coatings, as well as how higher storage test temperatures can produce misleading corrosion test results.
Coatings have physical properties, such as tensile strength and barrier strength that disappear when the coating temperature is at or above the coating’s Tg. In other words, a coating loses its physical properties when the temperature is above its Tg.
The internal coating for a spray package is dry before it is filled. Examples of dry coating Tg magnitudes are:
• Epoxies and polybutadiene are around 100°C (212°F)
• Nylon 6 is around 47°C (116.6°F)
• Nylon 6,6 is around 79°C (174.2°F)
• Polypropylene is around -10°C (14°F)
• PET has a Tg between 69°C (156.2°F) and 85°C (185°F), depending on the PET grade
Internal package coatings typically become wet shortly after the package is filled with product, because formula ingredients absorb into and diffuse through the coatings. Consequently, the internal dry coating Tg for a new, unfilled package is higher than the Tg for the same coating after the package is filled with product. For example, the Tg for a dry epoxy coating is 100°C (212°F), but the corresponding Tg for the same wet coating is around 50°C (122°F).
It is often assumed that a higher storage temperature will accelerate both the coating’s and the metal’s corrosion rates, thus allowing the time needed for storage stability corrosion tests to be reduced. This assumption is based on the Arrhenius equation that states chemical reaction rates double for every 10°C (50°F) increase in temperature.
However, metals and coatings do not follow the Arrhenius equation because the corrosion rates for both do not satisfy the two conditions for valid application of the Arrhenius equation:
1. The chemical reaction is first order
2. The chemical reaction rate is controlled by its energy of activation
In addition, metal corrosion is not a pure chemical reaction, but a combination of a chemical reaction (metal atoms change state to metal ions) with an electrical charge transfer between the metal and its environment. An environment can be either a formula or the permeate diffusing through a coating to the metal underneath.
Figure 1 illustrates how the Tg for a wet coating changes with increasing temperature. The metal corrosion rate under an epoxy coating is plotted on the Y-axis (log scale) and the corresponding temperature is plotted on the X-axis. The liquid-permeate in this case is water, and the coating was completely saturated.
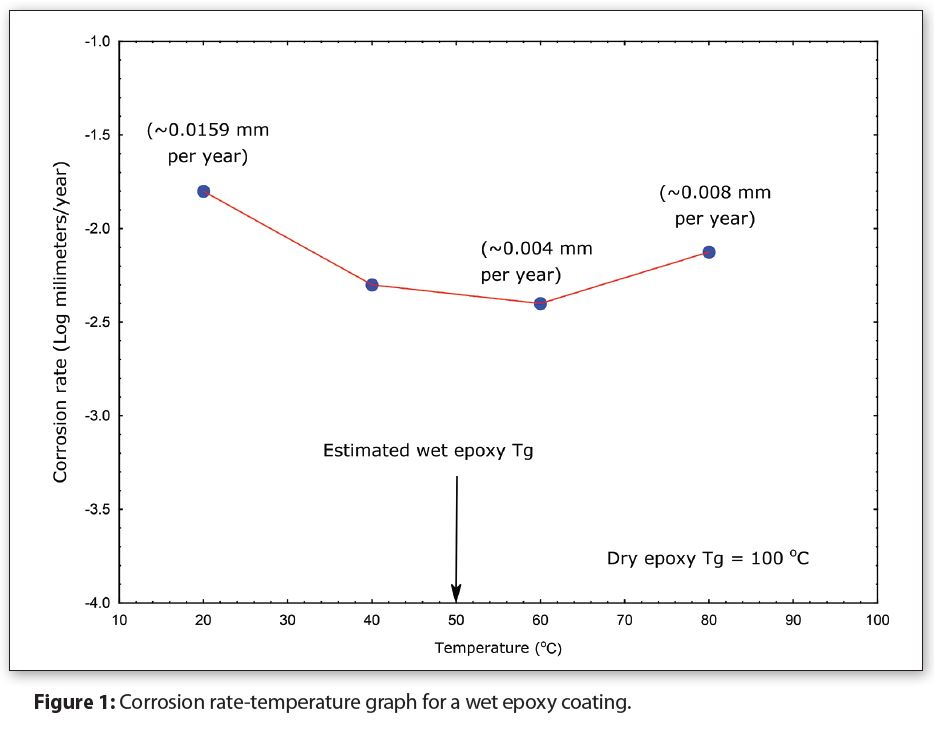
The inflection point for the graph is around 50°C (122°F), which is the most likely estimation for the wet epoxy coating Tg.
The corrosion rate in Figure 1 decreases from approximately 0.016mm per year at 20°C (68°F), to approximately 0.004mm per year at 60°C (140°F) (-1.8 and -2.3, respectively on the Y-axis). In this case, increasing the temperature actually decreases corrosion instead of increasing it, contrary to expectations from the Arrhenius equation.
In addition, the corrosion rate at 20°C (68°F) (0.0159 mm/year) is more than three times larger than the rate at 40°C (104°F) (0.005 mm/year), indicating that corrosion is worse at room temperature (20°C/68°F). This trend also contradicts the Arrhenius equation. I have actually seen numerous storage tests where the room temperature corrosion was worse than the higher temperature corrosion, and vice versa.
It’s tempting to say from Figure 1 that temperature accelerates corrosion as the temperature is increased above 50°C (122°F). However, the corrosion rate at 80°C (176°F) should actually be greater than 0.016mm per year instead of 0.008mm per year–also not consistent with the Arrhenius equation. Consequently, it’s more accurate to conclude that the wet coating is no longer a barrier when temperatures are above the 50°C (122°F) wet epoxy Tg.
Therefore, I typically do not recommend shortening storage test length with higher storage temperatures. Corrosion test length can be reduced with electrochemical measurements when the appropriate instruments, measurement parameters, exposure times, analysis protocols, sample size and data analysis and interpretation protocols are used.
Summary of Parts 1–3
Part 1: Coatings do not always prevent corrosion and are not always needed to prevent corrosion. Whether or not a coating is needed to protect a metal from corrosion is determined by a complex interaction between a formula’s chemical composition, the type of metal and the type of coating, as well as a variety of metal surface attributes that produce various coating defects.
Part 2: Defects in coatings are always present and typically not visible with either the unaided eye or a light microscope. Consequently, electrochemical measurements with sensitive instruments are needed to detect and measure if these defects will or will not cause package corrosion.
Part 3: The Tg of a coating decreases when it becomes wet. Absorption of formula ingredients into coatings cause them to become wet and subsequently degrades coating properties, such as its Tg. Raising storage temperatures to evaluate the corrosion resistance of coated metal packages does not reliably predict package corrosion in a smaller amount of time.
Thanks for your interest and I’ll see you next year. Contact me at 608-831-2076; rustdr@pairodocspro.com or from our two websites: pairodocspro.com and aristartec.com. SPRAY