Written on: August 1, 2015 by W. Stephen Tait

Most of the material defects in the previous two Corrosion Corners are very small, but can still be detected by the unaided eye. A microscope is needed, of course, to see the details of these small defects.
There are also material defects that can only be seen with a scanning electron microscope or a light microscope; these defects can also contribute to or cause spray package corrosion. Therefore, microscopic material defects in metals could also contribute to or cause spray package failure, such as leaking.
The metals used for spray packaging are not pure metals. Instead, they are alloys that are mixtures of metals and non-metals, such as carbon and oxygen.
Metal/non-metal compounds, such as metal oxides or metal carbides, fall out of solution and form microscopic particles in the bulk alloy as a molten alloy cools. These particles are dispersed throughout the alloy bulk and are referred to as inclusions. Inclusions can be flattened and elongated during the process of rolling and thinning the metal into the sheets for spray package fabrication and when the metal is extruded into a container.
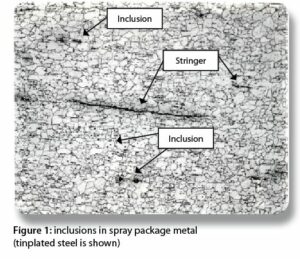 Figure 1 provides a photomicrograph with examples of inclusions. They are the small dark spots and the thin lines in the metal. The thin lines are also referred to as stringers. The arrows show the locations for only a few of the many, many defects in Figure 1.
Figure 1 provides a photomicrograph with examples of inclusions. They are the small dark spots and the thin lines in the metal. The thin lines are also referred to as stringers. The arrows show the locations for only a few of the many, many defects in Figure 1.Inclusions are sites for pitting corrosion and stress cracking. Stress cracking is rare, and the chemical composition of your formula determines whether or not pitting corrosion occurs at inclusions.
Please keep in mind that inclusions are not impurities. Inclusions are material defects that result from alloying a metal with different elements to obtain desirable properties, such as strength and formability.
Metals form regular arrangements of atoms, and these atomic arrangements form bulk formations referred to as crystal planes. Crystal planes are often not perfect, and there are often fragments of planes between complete planes. The fragment planes are referred to as dislocations.
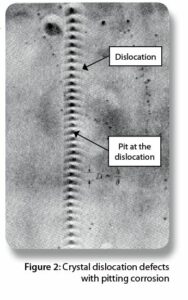 Figure 2 provides an example of dislocations and how they pile up when a metal is rolled into a sheet. Steel and aluminum metals and alloys all have dislocations.
Figure 2 provides an example of dislocations and how they pile up when a metal is rolled into a sheet. Steel and aluminum metals and alloys all have dislocations.
Figure 2 also shows that dislocations could be sites for the initiation of pitting corrosion. The solution that caused the pitting corrosion in Figure 2 was an etching solution of ethyl alcohol and nitric acid.
It has been estimated that there are approximately one million dislocations per square centimeter of metal surface. The chemical composition of your formula determines whether or not pitting corrosion occurs at dislocations, like those shown in Figure 2.
Figure 3 provides a scanning electron micrograph of tinplated steel. The tin coating was removed from the steel by mechanical polishing. The steel was further polished after the tin was removed and then etched with the alcohol/nitric acid solution previously mentioned.
There are a several different types of defects shown in Figure 3:
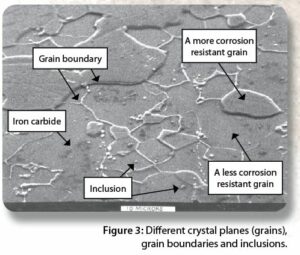 The material defects shown in Figure 3 can contribute to or cause spray package corrosion. Aluminum and steel both have one or more of the types of material defects shown in Figure 3.
The material defects shown in Figure 3 can contribute to or cause spray package corrosion. Aluminum and steel both have one or more of the types of material defects shown in Figure 3.
The chemical composition of your formula determines whether or not the material defects in Figure 3 contribute to or cause spray package metal corrosion. The current state of corrosion science on how and why corrosion initiates is not advanced enough to model and predict when corrosion will occur. Hence, corrosion testing is essential for reducing the risk that a given type of formula will contribute to or cause corrosion of the type of spray package chosen for your formula.
We would be happy to teach our Elements of Spray Package (Aerosol Container) Corrosion short course at your R&D facility. Want a specific topic discussed in Corrosion Corner? Please send any suggestions/questions/comments to rustdr@pairodocspro.com or visit www.pairodocspro.com. Back articles of Corrosion Corner are available from Spray. Thanks for your interest and I’ll see you in September.